introduction
The simultaneous removal of particles, nitrogen oxides and sulfur oxides from the flue gas can be achieved with catalyst-impregnated ceramic filter elements in a housing box system. Most of the pollution control equipment installed is dominated by 59% baghouse fabric filters and 39% ESPs. By using a 3-in-1 CCF process, investment costs of 10 to 20% can be achieved compared to conventional ESP / baghouse and SCR / SNCR configurations, as well as space savings. There are currently 74 glass factories in the United States consisting of 133 ovens, 42 of which are installed with air pollution control equipment. 25 of the sites are in operation or installing CCF units. The more facilities install or upgrade their units, the larger the CCF base becomes.
The three most common pollutants in the air we breathe are particulate matter (PM), nitrogen oxides (NOx) and sulfur oxides (SOx). Nitrogen dioxide (NO2) is a highly reactive gas or an oxide of nitrogen (Nox). Oxides of nitrogen include nitrous acid and nitric acid. Common types of NOx removal devices are Selective Catalytic Reduction (SCR), which uses a catalyst to react with ammonia at about 700 ° F, and Selective Non-Catalytic Reduction (SNCR), which reacts with ammonia at higher temperatures to produce NOx to reduce.
The United States Environmental Protection Agency (USEPA) regulates all three pollutants. These three main sources of pollution can cause breathing difficulties and asthma, as well as environmental damage caused by acid rain, impaired vision and effects on water quality.
NOx treatment
The catalytic reduction of NOx takes place in a typical temperature range between 600 to 700 ° F. The NOx reduction process is performed by injecting an ammonia based reagent.
In 1150 ° F high temperature furnace exhaust applications, water spray injection, fresh air dilution, or heat exchangers can be used to cool and control the gas velocity and temperature distribution to achieve the optimal reaction conditions.
SOx treatment
One group of sulfur oxides (SOx), mainly SO2, is of concern due to the formation of sulfuric acids. Wet flue gas desulphurisation (WFD), circulating dry scrubbers (CDS) and semi-dry scrubbers (SDA) are usually used for SOx removal.
The lime reacts with SO 2 in operating temperature ranges between 350 ° F and 1200 ° F to form salt and water in the following reaction using calcium (hydrated lime) and sodium-based sorbents (sodium bicarbonate or trona).
PM treatment
Particulates are a mixture of solid particles, liquid droplets, dust, dirt, soot, and smoke (in the 2.5 to 10 microns range) that include bases, acids, chemicals, liquids, solids, metal dust, and gas particles that are common with the baghouse – Remove the fabric filter or a wet or dry electrostatic precipitator (ESP, wetESP). Both limit values for particles (PM) such as PM10 (<10 micrometers) and PM2.5 (less than 2.5 micrometers) are regulated.
Multi-Polltant Control: 3 in 1
As shown in Figure 3, there are three common methods (SCR, SDA, Baghouse) in the industry for removing NOx, SOx and PM. The various technologies and devices have been successfully used to control pollutants. The advantage of using ceramic catalytic filters is that less equipment is used, thus reducing the space required on site. Figure 8 summarizes the removal efficiencies of the CCF unit for various pollutants.
Illustration 1: ESP vs. CCF vs. baghouse fabric filter. A single CCF unit achieves the same removal efficiencies with less space requirements and lower operating and maintenance costs.
Housing made of ceramic catalytic filter (CCF)

Figure 2: Flue gas treatment system with FLKCAT filter elements in 5 filter boxes.
Fabric filters (also called baghouses) and ceramic filters can absorb over 99.9% of the particles. including PM2.5. In combination with a sorbent injection, these become multi-pollutant systems Remove additional pollutants like mercury, SOx and other acidic gases. NOx can be removed via SCR catalysts built into the filter elements and upstream injection of ammonia or, if the Temperature is high enough, urea.
Ceramic filters are also called candle filters because of their massive tube shape. The low density ceramic filters can be with or without an embedded catalyst.
The use of catalytic ceramic filter box systems enables multiple pollutants to be controlled in one step. It has been found that the advantages of such a system are;
- CAPEX savings: Less equipment for the PM
- Removal of dioxins, heavy metals
- Reduced utility usage. Lower pressure drop throughout the system
- Operating cost savings, reduced maintenance costs, less equipment to maintain.
- Fewer moving parts
- Highly efficient pollutant removal
- Can run at high exhaust temperatures. (> 350 ° F)
- Modular structure: One filter box can be serviced without interrupting operation.
- 5 to 10 years service life of the catalyst filter elements.
- The filter cake is removed by automatic online pulse jet cleaning
- Operation takes place outside of acid and water dew points
Ceramic candle filter
Candle-shaped ceramic filters are a rigid tube with a porosity of 80-90%, light refractory fibers and organic and inorganic binders. Dispersants, pore formers, high temperature binders, and plasticizers are typically added to make the filter tube, which enables excellent heat resistance and thermal shock resistance. Outlets with a high internal surface due to the fiber structure. The filters are most commonly supplied in lengths of 10 feet by 6 inches in diameter and weighing 12.5 kg (27 lbs).
See Figure 7 for detailed dimensions. Some suppliers are investigating longer lengths of 20 feet, which will allow fewer items per box and therefore even smaller footprints.
Low density ceramic filters made from fibers ~ 3 µm in diameter are vacuum formed from a fiber slurry into a rigid tubular fibrous filter element. The filter elements are installed as an alternative to conventional pulse jet cleaned fabric filter elements. The filter elements are self-supporting via an integrated flange, as shown in Figures 3, 4 and 5. The pressure drop across the filter box is kept at 4 ”when in clean condition.
Dry sorbents based on calcium or sodium are injected into the flue gas in front of the ceramic filter SO2 removal. Activated charcoal or brominated charcoal could also be injected to remove them Mercury. The flue gas is drawn through the filter tube wall by an ID fan, where it is collected Particles form as cakes on the outside of the tube (see Figure 6). NOx is removed catalytically Reduction with injected ammonia or urea with the formation of nitrogen and water; the catalyst is embedded in it the filter walls. The clean flue gas then enters the air chamber through the pipe. The cake is made on a regular basis cleaned online from the filter walls using the standard pulse jet process. Optimal pulse beam frequency and pressure improve NOx reduction.
The ceramic filters are spray bedded and dried with a catalyst slurry to allow reactions to remove NOx. The catalytic filter element consists mainly of Al2O3, SiO3, TiO2, V2O5 and WO3. The micro-sized catalyst particles are distributed over the entire wall thickness, creating a large catalytic surface. Their microporous structure and small size partly explains the increased reactivity at lower temperatures.
Catalytic ceramic filters have a small footprint and are easy to use. You could replace an existing ESP and eliminate the need for a separate SCR unit. They could be retrofitted in industrial plants where there is not enough space for a conventional SCR unit.
The modular design of the box housing units enables the configuration of filters for large gas flows Volumes. The systems can be designed in such a way that a single box can be removed from the network if necessary, and
the remaining two or more boxes continue to work at a slightly higher pressure without interrupting the process itself and without any significant change in emissions.
No water is used and therefore no wastewater is produced. However, the fly ash must be disposed of. The catalytic ceramic filters must be disposed of at the end of their service life. The catalyst life has been found to be longer than conventional high-dust SCR Systems. The typical lifespan of ceramic filters is five to ten years, with some installations having a lifespan of more than 10 years. Ceramic catalytic filters have not yet been used in high dust applications such as those found in coal-fired power plants and cement plants.
Installation of filters

Figure 3: Installed catalytic filter elements from FLK Ceramic
Figure 4: Installation of FLK ceramic catalytic filter elements
Figure 5: Installed catalytic filter elements from FLK Ceramic 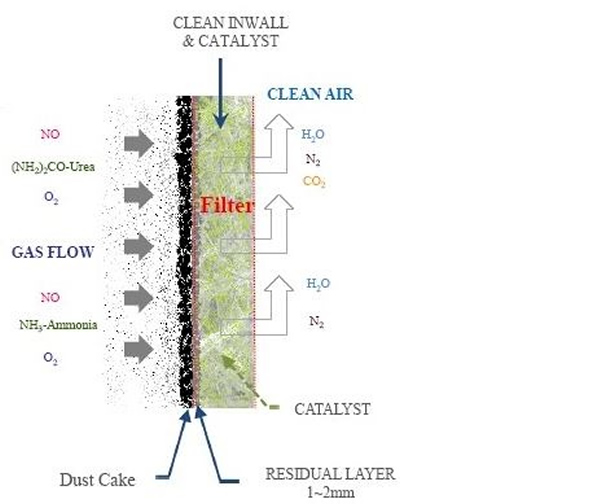
Figure 6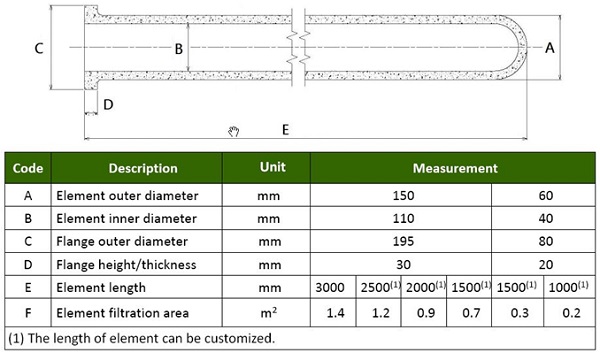
Figure 7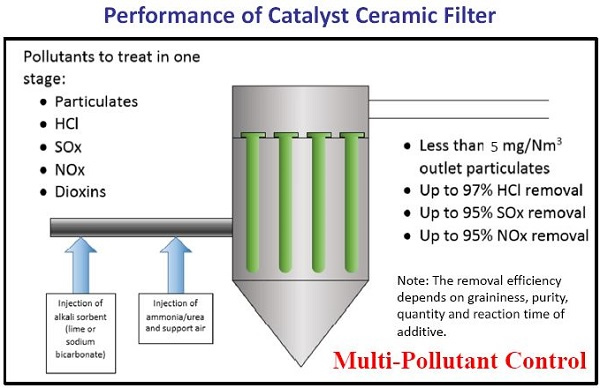
Figure 8. Summary of pollutant removal performance
REFERENCES
Carpenter Anne M (2013) Advances in multiple pollutant control; IEA Clean Coal Center
Heidenreich S (2013) Hot gas filtration – a review. fuel;; 104;; 83-94 (February 2013)
Moos KD (2011) Advanced multipollutant control with lightweight ceramic filters. Air pollution control;; 1(3); 15-19 (June 2011)
Moos KD (2012a) Ceramic filter systems. Ceramics industry;; 23-27 (June 2012)
Moss K (2012b) Advanced air pollution control in one system. Pollution technology;; 12-16 (January 2012)
Startin A, Elliott G (2009) Emission control with ceramic filters. Chemical engineering;; 35-39 (January 2009)
